FAQs
From technical queries, ordering and delivery to access details and more. If you can’t find the answers you’re looking for, please CONTACT US.
Questions about the CAR-T cell therapy process?
-
Does the treatment center qualification expire? If so, when does it expire?
Center qualification does not expire, however re-training (including but not limited to apheresis collection, product receipt, infusion and Risk Management Plan), as well as contract review, may be required depending on the duration of order inactivity. Country-specific regulations should also be considered.
Center qualification does not expire, however re-training (including but not limited to apheresis collection, product receipt, infusion and Risk Management Plan) may be required and will be conducted depending on the duration of order inactivity. Country-specific regulations should also be considered.
Certification
-
How can I grant access to the ordering platform for a new user at my treatment center?
Users must be part of a certified/qualified treatment center and complete the required training to access the platform. Please contact Customer Service Operations to request training and account setup for new users.
-
Who from my treatment center can place an order?
Novartis CAR-T products must be prescribed by a qualified healthcare professional at your treatment center. Authorized individuals on the Patient Care Team can submit an order on their behalf.
Novartis CAR T cell products must be prescribed by a qualified prescriber at your treatment center. Authorized individuals on the patient care team can submit an order on behalf of the qualified prescriber.
-
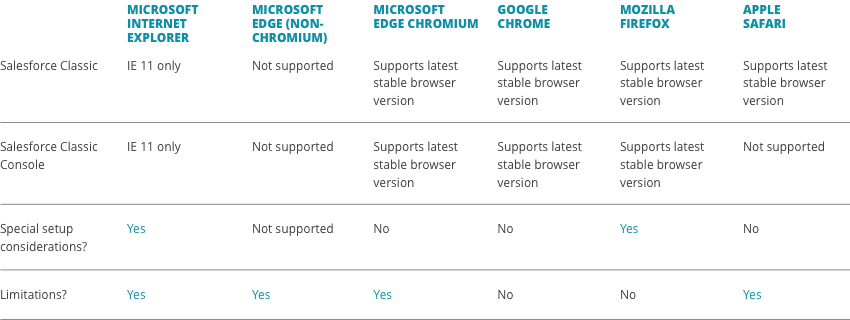
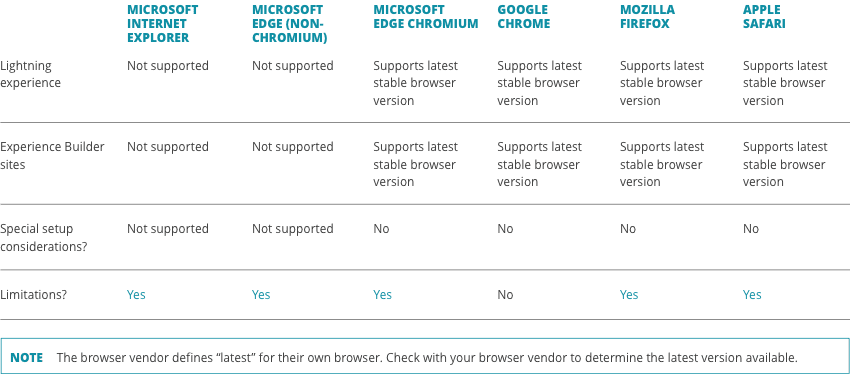
What are the system requirements to use the CellChain™ order management platform?
Most browsers are compatible with CellChainTM, but please refer to the recommended browser requirements below for both platforms.
CellChain™ Classic
CellChain™ Pro
-
Our hospital has not been upgraded to CellChain™ Pro yet; when can I expect this to happen?
Please contact your local Novartis representative who will be able to assist you with an estimated upgrade timeframe.
-
Will I know when the final product will be shipped?
The current estimated final product delivery date will be reflected in CellChain™. Both the treating physician and cell processing lab will receive an email from CellChain™ when tisagenlecleucel has been shipped.
-
How many finished product bags will I get?
One dose may be contained in up to three bags of finished product. When you receive the product, you can verify the number of bags received on the Certificate of Conformance (CoC) and Certificate of Analysis (CoA).
-
What should I do if I receive multiple final product bags?
If more than one bag has been received for the treatment dose, thaw only one bag at a time. Wait to thaw the next bag until it is determined that the previous bag has been safely administered. Please refer to the Prescribing Information for details.
-
Can you hold product delivery back for a bit?
Novartis will ship tisagenlecleucel once it is ready, but the treatment center can store the cryopreserved product until its expiration date, which is 9 months after the manufacturing date. If there are unique circumstances that require alternate arrangements, Customer Service Operations can assist.
Order related
-
My hospital is not certified to treat with CAR-T treatments, where can my patient be assessed for CAR-T treatment?
There are more than 350 REMS-certified CAR-T treatment centers located worldwide. To find a qualified treatment center, please click here.
Please note this is not an exhaustive list of treatment centers, and there may be additional centers not listed here. If you are unable to find a suitable center, please contact our Customer Service Operations team who will be able to assist. -
CAR-T treatment is not approved in my country, can my patient be assessed for CAR-T treatments?
To obtain information on how to provide access to CAR-T treatments for your patient please visit: mytcelltherapies.com
Access
-
Why do you require cryopreservation?
Cryopreservation provides flexibility for appropriate patients' cells to be collected at the optimal time based on their health condition. Novartis can accept apheresis material that was collected within 30 months for commercial manufacturing.
Apheresis
Questions about the CAR-T cell therapy process?
Try our CellCompass customer service bot to get you the answers.

